While you can – listen to the CEO of Pfizer. After once claiming his shots are “100 effective,” Pfizer CEO now says two COVID shots “offer very limited protection, if any,” against COVID-19. It’s something anyone in the real world can attest to as most Omicron cases are now occurring in people twice or three times vaccinated.
They have deleted THIS video? pic.twitter.com/rN1inCdsxo
— Ernst Stavro (@OliL73) January 11, 2022
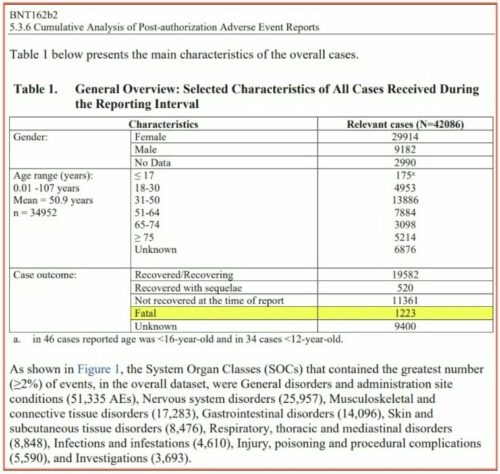
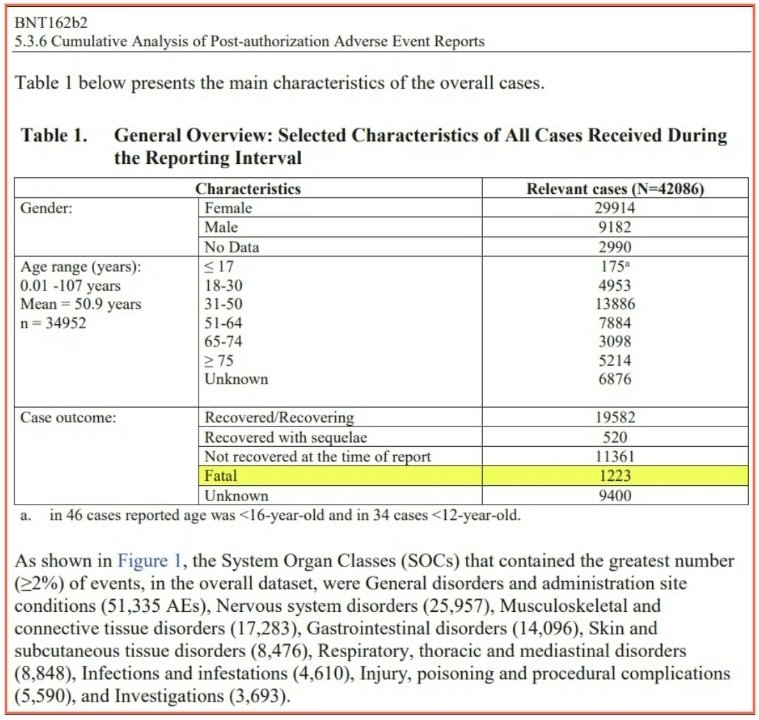
This is Pfizer’s own data. This is the link to the full report published below: https://phmpt.org/wp-content/uploads/2021/11/5.3.6-postmarketing-experience.pdf
Among adverse event reports received into the Pfizer safety database during the cumulative period, only those having a complete workflow cycle in the safety database (meaning they progressed to Distribution or Closed workflow status) are included in the monthly SMSR. This approach prevents the inclusion of cases that are not fully processed hence not accurately reflecting final information. Due to the large numbers of spontaneous adverse event reports received for the product, the MAH has prioritised the processing of serious cases, in order to meet expedited regulatory reporting timelines and ensure these reports are available for signal detection and evaluation activity. The increased volume of reports has not impacted case processing for serious reports, and compliance metrics continue to be monitored weekly with prompt action taken as needed to maintain compliance with expedited reporting obligations. Non-serious cases are entered into the safety database no later than 4 calendar days from receipt. Entrance into the database includes the coding of all adverse events; this allow for a manual review of events being received but may not include immediate case processing to completion. Non-serious cases are processed as soon as possible and no later than 90 days from receipt. Pfizer has also taken a multiple actions to help alleviate the large increase of adverse event reports. This includes significant technology enhancements, and process and workflow solutions, as well as increasing the number of data entry and case processing colleagues. To date, Pfizer has onboarded approximately (b) (4) additional full- time employees (FTEs). More are joining each month with an expected total of more than (b) (4) additional resources by the end of June 2021.
Curious how many died during the Pfizer reporting interval?